Signed in as:
filler@godaddy.com
Signed in as:
filler@godaddy.com
Smartphones and tablets have become ubiquitous; most people carry at least one highly capable computer with them everywhere they go and have cloud access to high powered central computers. As technology advances all aspects of health care, software has become an essential part of all products, integrated widely into digital platforms. Software, which on its own is a medical device – Software as a Medical Device (SaMD) – is one of three types of software related to medical devices. The other two types of software related to medical devices include software in a medical device and software used in the manufacture or maintenance of a medical device.
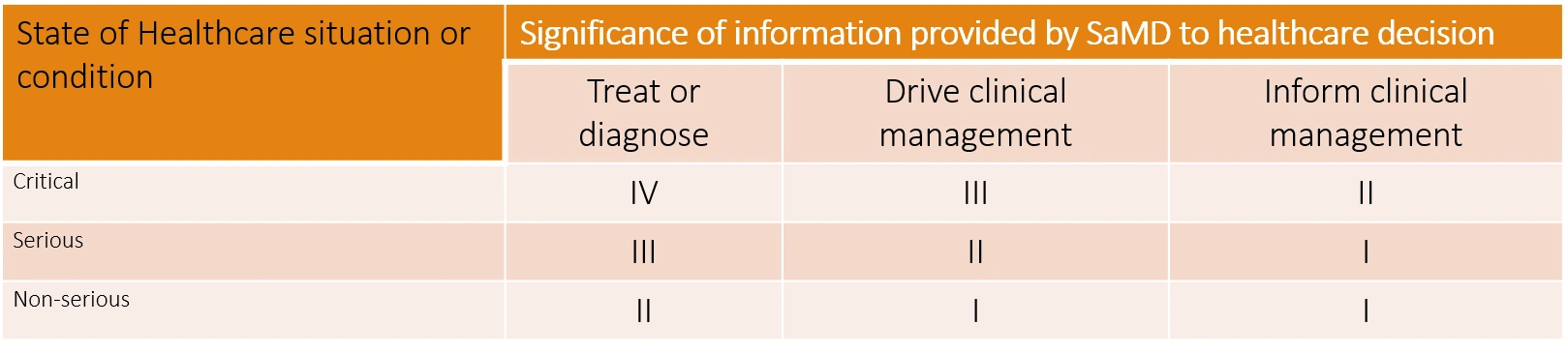
“Software as a Medical Device” (SaMD) is broadly defined as software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.

Whether using a predictive (Waterfall; V- model) or adaptive methodology (Agile), it is imperative that the following processes and their corresponding outputs to be incorporated in the SaMD Development:
Manufacturers of SaMD must report any correction or removal of an SaMD if action was initiated to:
We have the regulatory savvy, QMS experience, and technical expertise to help mature and emerging technology companies meet their challenges and take full advantage of their business opportunities.
“Software as a Medical Device” (SaMD) has been defined as software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.
What is needed to create and launch an SaMD?
Be able to answer the following questions:

This website uses cookies. By continuing to use this site, you accept our use of cookies.